Table of Contents
In this article, you will find a handful of information about the stability of iodine in a saltwater aquarium. We conducted a series of studies using ICP-OES to determine the effect of light on its stability in the tank.
Where did this idea come from? For every saltwater aquarist, iodine is a kind of challenge. It is no secret that the stability of parameters in a saltwater aquarium is important, if not the most important. In artificial conditions, this is quite a difficult task, especially when you are just starting out with saltwater aquariums. Seawater involves many dependencies and interconnections between elements. It is worth knowing how to dose iodine, especially if your aquarium inhabitants are corals that have a high demand for this microelement.
Basic information about iodine, its importance for marine animals, the effects of iodine deficiency or excess in the aquarium, and recommendations for its dosing can be found in a separate article – Iodine in a saltwater aquarium and its importance.
However, it is worth remembering the effects of both iodine deficiency and excess in the tank, so I will remind you here:
Iodine deficiency
- causes health problems in fish and some invertebrates,
- corals can become more susceptible to parasites,
- corals expel zooxanthellae,
- coral tissue becomes dull,
- there is also a noticeable inhibition of coral growth.
Excess iodine
- Is toxic to organisms living in the aquarium, which can lead to diseases and death,
|
The toxicity of iodine depends on several factors, with the most important being:
|
- Corals become darker,
- Increased algae growth.
Key factors influencing iodine stability
Iodine stability in a saltwater aquarium depends on many factors. Below are a few key ones that significantly influence iodine fluctuations:
- pH of the water – a high pH promotes oxidation reactions, so in seawater, we primarily find iodates alongside iodide and iodine.
- chemical reactions – iodine can react with other substances in the water, transforming into forms that are less accessible to living organisms.
- water changes – altering water parameters by using iodine-poor or iodine-rich water.
- activated carbon – using carbon absorbers to remove chemical pollutants poses a risk of also removing iodine.
- UV radiation – light accelerates the oxidation of iodine (this is unavoidable in marine aquariums).
- changing animal population – every aquarium is unique, and the needs of marine animals vary, affecting their consumption of iodine.
Ways to control iodine levels in an aquarium
Controlling iodine levels is not easy. Elemental iodine primarily forms numerous ionic compounds in saltwater. Free iodine (I2) is rarely found in saltwater because it quickly transforms into oxidized forms such as iodates.
Measuring iodine levels at home is difficult because the correct level is around 10-6 grams per liter of saltwater. Drop tests are available on the market, but in my opinion, they are not very precise for iodine.
More advanced analytical techniques are used to measure iodine in saltwater, such as:
- Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) is a technique used for analyzing iodine in saltwater aquariums.
- Ion Chromatography (IC) is a less commonly used method for analyzing iodine in marine aquariums.
How is the iodine level measured using ICP-OES
In short, measuring iodine in seawater using ICP-OES involves detecting the radiation emitted by excited iodine atoms in the inductively coupled plasma. This technique is considered the most accurate for determining iodine content in marine environments.
Iodine content measured with ICP-OES is typically expressed in units such as:
- milligrams per liter (mg/L)
- parts per million (ppm)
- micrograms per liter (µg/L)
For example, a result of 0.07 mg/L can be expressed in three ways:
0,07 mg/L = 0,07 ppm = 70 µg/L
Challenges in measuring iodine using ICP-OES
It’s worth knowing that the ICP-OES technique, although extremely accurate and precise, has its limitations.
- Despite its ability to measure the total iodine content in a sample, ICP-OES does not allow for the identification of individual chemical forms of this element:
- I2 (elemental iodine),
- I– (iodide form),
- IO3– (iodate form),
- Organic forms of iodine.
- *Interference in analysis means that the presence of iodine can affect the accuracy of measuring other elements, such as phosphorus, which can lead to incorrect measurement results. What does that mean?
If you have a high level of phosphates in your tank, accurately measuring iodine using ICP-OES becomes difficult. Don’t worry, experienced chemical analysts are aware of this possibility and know methods that allow for accurate determination of both parameters simultaneously. - Iodine is difficult to ionize (remove an electron from), as it requires a significant amount of energy to initiate the ionization process. All these factors together result in iodine having low ionization efficiency, which complicates its analysis in many analytical techniques, including ICP-OES.

- A water sample sent to the laboratory should arrive as quickly as possible because external factors and the matrix of seawater can affect the level of iodine. This element is not stable over time, which is a common issue in chemical analysis (iodine degradation). To minimize iodine degradation in the sample, I recommend:
-
- fill the vial to the top – this limits oxygen access, which accelerates iodine degradation.


- fill the vial to the top – this limits oxygen access, which accelerates iodine degradation.
-
- avoid high temperatures – I may not always control shipping conditions, but try not to hold the water vial at home for long before sending it via courier.
- avoid exposing the vial to light – iodine is sensitive to light, which can cause it to degrade.
How and when to dose iodine?
How to dose iodine in a saltwater aquarium?
When caring for a saltwater aquarium, iodine is typically added regularly to maintain an optimal level of 70 μg/L for marine organisms.
However, there are actually two schools of thought regarding iodine:
- Continuous dosing (Iodine Reef Minerals or KH smart component).
- No additional supplementation (water changes and other products containing iodine).
| Regardless of which school you choose, it is important to maintain a proper and stable level of iodine in the aquarium. |
When is the best time to dose iodine – after ICP tests?
I have observed how iodine behaves in my aquarium under two conditions:
- Daytime mode – for two weeks, iodine was dosed in the morning, just before turning on the light.
- Nighttime mode – for the following two weeks, iodine was dosed at night, shortly after turning off the light.
Basic aquarium data and light settings
Description of the aquarium
- Tank dimensions: 50x60x40 cm (120 liters)
- Water volume in circulation: 90 liters
- Sump: None
- Rock: Ceramic + live rock
- Lighting: Reef Factory Reef flare PRO
- Livestock: Mixed
- Fish: 2 x Pterapagon and 2 x Clownfish
The spectrum of the light settings during the test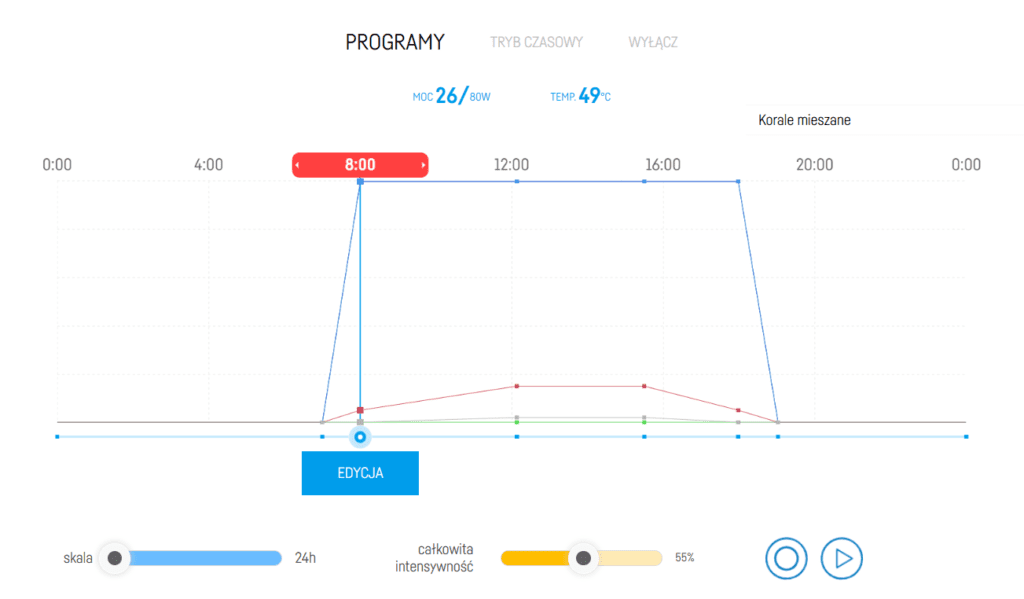
Dosing iodine
During the testing phase, a single dose increased iodine levels by approximately 14 µg/L. Iodine was precisely dosed using an automatic pipette before the circulation pump. Reef Factory’s product, Iodine Reef Minerals, was used for dosing (10 ml of the product increases levels by 0.2 ppm or 200 µg/L in a 100-liter saltwater aquarium).
I: Daytime mode: 14-day testing period
Sample taken before iodine dosing – ICP test
Iodine dosed in the MORNING with Iodine Reef Minerals, before turning on the light – ICP test
Sample taken after iodine dosing – ICP test
Sample taken after turning off the light on the same day – ICP test
II: Nighttime mode: 14-day testing period
Sample taken before iodine dosing – ICP test
Iodine dosed in the EVENING with Iodine Reef Minerals, after turning off the light – ICP test
Sample taken after iodine dosing – ICP test
Sample taken after turning on the light the next day – ICP test
To avoid overdosing iodine during the testing period, I stopped dosing Smart Components and supplemented the drop in main parameters using products from the Reef Minerals series. This decision was made because SC KH contains iodine.
Results of ICP measurements
Samples collected were analysed on ICP-OES at the Reef Factory laboratory no later than 48h after sampling (average time was 30h).
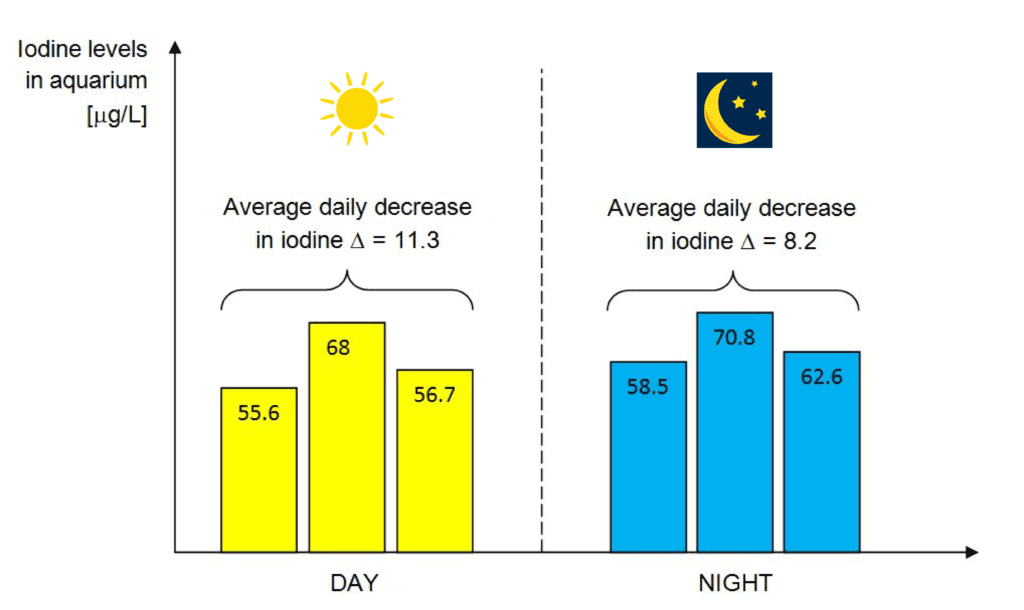
In the tested aquarium, the average daily decrease in iodine was 11.3 µg/L in the daytime mode and 8.2 µg/L in the nighttime mode. These losses were compensated by daily iodine dosing. However, a more pronounced downward trend was observed when dosing iodine just before turning on the light (daytime mode). These differences are slight, so it cannot be conclusively stated that dosing in one mode is less effective than the other.
Photodegradation (degradation due to light exposure) of iodine is significant, and the marine aquarium environment enhances such processes. Iodine levels can significantly decrease within a few days. In addition to light, there are other factors influencing the rate of iodine degradation.
In my opinion, the regularity of dosing is more important than deciding whether to dose iodine at night or in the morning.
Summary
Maintaining a stable level of iodine in a saltwater aquarium is crucial but also challenging. Iodine plays a significant role in the health of marine organisms, especially corals, which have a high demand for this micronutrient. Research has shown that the average daily decrease in iodine in the tested aquarium was 11.3 µg/L in daytime mode and 8.2 µg/L in nighttime mode. Photodegradation of iodine due to light exposure is evident, but it’s important to note that there are other factors influencing its stability. The regularity of iodine dosing is key, and the time of day when it is added to the aquarium is less critical.
*The presence of one substance can interfere with or affect the accuracy or results of measurements of another substance.
About author

Magdalena Metzler
Privately, I am a mother and a lover of nature and sport. My main interest is quantum chemistry, which hides a whole lot of unsolved mysteries and connections, which is extremely exciting from a scientific point of view.
In my scientific career, I have conducted international projects focused on innovative solutions for many branches of business, e.g. automotive, construction, and now, of course, marine aquaristics.
Working at Reef Factory gave me a passion for marine aquaristics, which I can develop every day, building a chemistry department and creating products that will help aquarists take care of tanks and ensure the highest safety of animals. One of the most exciting memories of working at Reef Factory is the commissioning of the ICP-OES spectrometer, which analyzes the elemental composition of seawater. The method of analysis in ICP is based on an analytical technique, which is a combination of my passion for quantum chemistry and marine aquaristics.
I hope you find my articles on ReefPedia interesting and helpful! Happy reading :))